San Joaquin Valley Fever is a human disease caused by the fungus, Coccidioides immitis. This fungus and it’s sister species, Coccidioides posadasii, are found in hot, dry regions from the San Joaquin Valley of California, through Southern California, Arizona, New Mexico, Texas, Mexico, Central America and South America. We have studied how it reproduces (Burt et al. 1996), evidence for its recent speciation (Koufopanou et al. 1997; Fisher et al. 2002), presence of populations within each species (Fisher et al. 2001), genetic exchange among species that shows evidence of natural selection of key genes (Neafesy et al. 2010) , and how its genome reveals an evolutionary to switch from using plants to using animals for nutrition (Sharpton et al. 2009). These studies have led us to propose that the fungus is closely associated with wild, native rodents and that it relies on the rodents for the food and water that it needs to reproduce (Taylor and Barker, 2019).
Currently, we are challenging our proposal about the intimate association of Coccidioides immitis and native rodents and the effect that agricultural and urbanization has on this association by sampling for the fungus in undisturbed areas where rodent populations are intact, as well as agricultural and urban areas were native rodent populations are disturbed or absent.
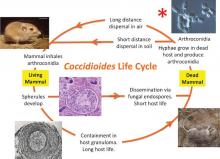
A life cycle of the Valley Fever fungus, Coccidioides immitis that emphasizes the close association of the fungus with wild
rodents, both in life and death. Rodents that survive initial infection make granulomas that constrain the living fungus in their
lungs. When the rodent dies of any cause, granulomas release the fungus, which digests the rodent to produce spores. The
fungus-rodent symbiosis evolved over millions of years before our arrival just 15,000 years ago. Not surprisingly, we exhibit the
same responses to infection, either making granulomas that constrain the disease, or suffering dissemination that requires
medical intervention. Taylor and Barker. 2019. Medical Mycology https://doi.org/10.1093/mmy/myy039
The direct effect of climate and soil on Coccidioides abundance are likely to be weaker than indirect effects that increase rodent food
and rodent populations. Taylor and Barker 2019 Medical Mycology. https://doi.org/10.1093/mmy/myy039
Publications on Coccidioides
- Taylor, J. W., & Barker, B. M. (2019). The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Medical mycology, 57(Supplement_1), S16-S20. https://doi.org/10.1093/mmy/myy039
- Taylor, J.W., Hann-Soden, C., Branco, S., Sylvain, I., Ellison, C.E. 2015. Clonal reproduction in fungi. Proceedings of the National Academy of Science 112(29): 8901-8908.
- Whiston, E. and J. W. Taylor. 2016. Comparative phylogenomics of pathogenic and non-pathogenic species. G3: Genes, Genomes, Genetics 6:235-244
- Taylor, J. W. 2015. Evolutionary perspectives on human fungal pathogens. Pp 23-40, In: Casadevall, A., Mitchell, A., Berman, J., Kwon-Chung, K., Perfect, J., Heitman, J. (eds), Human Fungal Pathogens, Cold Spring Harbor Perspectives in Medicine, CSHL Press, Cold Spring Harbor, NY. DOI: 10.1101/cshperspect.a019588
- Thompson, G. R., Stevens, D. A., Clemons, K. V, Fierer, Johnson, R. H., Sykes, J. Rutherford, M. Peterson, J. W. Taylor and V. Chaturvedi (2015). "Call for a California Coccidioidomycosis Consortium to Face the Top Ten Challenges Posed by a Recalcitrant Regional Disease." Mycopathologia 179: 1-9. https://doi.org/10.1007/s11046-014-9816-7
- Whiston, E., Taylor, J.W., 2014. Genomics in Coccidioides: Insights into evolution, ecology, and pathogenesis. Medical Mycology. 52, 149-155. https://doi.org/10.1093/mmy/myt001
- Wise, H-Z, Hung, C-Y, Whiston, E, Taylor, JW, Cole GT. 2013. Extracellular ammonia at sites of pulmonary infection with Coccidioides posadasii contributes to severity of the respiratory disease. Microbial Pathogenesis 59-60: 19-28. https://doi.org/10.1016/j.micpath.2013.04.003
- Whiston, E., Wise, H.-Z., Jui, G., Sharpton, T. J., Cole, G. T., J. W. Taylor. 2012. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS One 7: e41034. https://doi.org/10.1371/journal.pone.0041034
- Neafsey, D. E., B. M. Barker, Sharpton, T.J., Stajich, J. E., Park, D. J., Whiston, E. Taylor, J.W., Rounsley, S. D. (2010). Population genomic sequencing of Coccidioides fungi reveals recent hybridization and a novel form of transposon control. Genome Research 20:938-946. 10.1101/gr.103911.109
- Sharpton TJ, Stajich JE, Rounsley SD, Gardner MJ, Wortman JR, Jordar VS, Maiti R, Kodira CD, Neafsey DE, Zeng Q, Hung C-Y, McMahan C, Muszewska A, Grynberg M, Mandel MA, Kellner EM, Barker BM, Galgiani JN, Orbach MJ, Kirkland TN, Cole GT, Henn MR, Birren BW, and JW Taylor. 2009. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Research 19 1722-1731. 10.1101/gr.087551.108
- Johannesson, H. Kasuga, T., Schaller, R.A, Good, B. Gardner, M.J., Townsend, J.P., Cole, G.T., and J. W. Taylor. 2006. Phase-specific gene expression underlying morphological adaptations of the dimorphic human pathogenic fungus, Coccidioides posadasii. Fungal Genetics and Biology 43:545-559. https://doi.org/10.1016/j.fgb.2006.02.003
- Johannesson, H., Townsend, J.P., Hung, C.-Y., Cole, G.T., and J.W. Taylor. 2005. Concerted evolution in the repeats of an immunomodulating cell surface protein, SOWgp, of the human pathogenic fungi Coccidioides immitis and C. posadasii. Genetics 171: 109-117. 10.1534/genetics.105.040923
- Johannesson, H., Vidal, P., Guarro, J., Herr, R.A., Cole, G.T., and J. W. Taylor. 2004. Positive directional selection in the proline-rich-antigen (PRA) gene among the human pathogenic fungi Coccidioides immitis, Coccidioides posadasii and their closest relatives. Molecular Biology and Evolution 21:1134-1145.
- Fisher, M.C., Ranalla, B., Chaturvedi, V. and J. W. Taylor. 2002. Disease surveillance in recombining pathogens: multilocus genotypes identify sources of human Coccidioides infections. Proc. Natl. Acad. Sci. (USA) 99:9067-9071.
- Fisher, M. C., Koenig, G. L., White, T. J., and J. W. Taylor. 2002. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94:73-84. https://doi.org/10.1080/15572536.2003.11833250
- Koufopanou, V., Burt, A., Szaro, T., and Taylor, J. W. 2001. Gene Genealogies, Cryptic Species, and Molecular Evolution in the Human Pathogen Coccidioides immitis and Relatives (Ascomycota, Onygenales). Molecular Biology and Evolution 18: 1246-1258. https://doi.org/10.1093/oxfordjournals.molbev.a003910
- Fisher, M. C., Koenig, G. L., White, T. J., San-Blas, G., Negroni, R., Gutierrez Alvarez, I., Wanke, B., and J. W. Taylor. 2001. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc. Natl. Acad. Sci. (USA) 98:4558-4562.
- McEwen, J.G., Taylor, J.W., Carter, D., Xu J., Felipe, M.S.S., Vilgalys, R., Mitchell, T.G., Kasuga, T., White, T. and C.M.S. Soares. 2000. Molecular typing of pathogenic fungi. Medical Mycology 38 (Supplement 1): 189-97. https://doi.org/10.1080/mmy.38.s1.189.197
- Burt, A., Koufopanou, V., Taylor, J.W. 2000. Population genetics of human-pathogenic fungi. Pp. 229-244 In: Molecular Epidemiology of Infectious Diseases, R.C.A. Thompson (ed.). Arnold, London.
- Fisher, M.C., Koenig, G., White, T.W., Taylor, J.W. 2000. A test for concordance between the multilocus genealogies of genes and microsatellites in the pathogenic fungus Coccidioides immitis. Molec. Biol. Evol. 17:1164-1174. https://doi.org/10.1093/oxfordjournals.molbev.a026399
- Fisher, M.C., Koenig, G.L., White, T.J. and J.W. Taylor. 2000. Pathogenic clones versus environmentally driven population increase: analysis of an epidemic of the human fungal pathogen Coccidioides immitis. J. Clin. Microbiol. 38:807-813. https://jcm.asm.org/content/38/2/807
- Greene, D. R. and Taylor, J. W. 2000. Soil isolation and molecular identification of Coccidioides immitis. Mycologia 92: 406-410. https://doi.org/10.1080/00275514.2000.12061175
- Fisher, M., White, T. and J.W. Taylor. 1999. Primers for genotyping single nucleotide polymorphisms and microsatellites in the pathogenic fungus Coccidioides immitis. Mol. Ecol. 6:1082-1084.
- Burt, A., Dechairo, B.M., Koenig, G.L., Carter, D.A., White, T.J., and J.W. Taylor. 1997. Molecular markers reveal differentiation among isolates of Coccidioides immitis from California, Arizona and Texas. Mol. Ecol. 6:781-786. https://doi.org/10.1080/15572536.2003.11833250
- Koufopanou, V., Burt, A. and J.W. Taylor. 1997. Concordance of gene geneologies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc. Natl. Acad. Sci. 94:5478-5482. Correction 1998, PNAS 95:8414
- Bowman, B. H., T. J. White, and J. W. Taylor. 1996. Evolutionary relationships of human pathogenic fungi: multiple origins of pathogenicity in the fungal order Onygenales. Molec. Phylog. Evol. 6:89-96.
- Burt, A., D. A. Carter, G. L. Koenig, T. J. White, and J. W. Taylor. 1996. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis (Ascomycota). Proc. Natl. Acad. Sci. 93:770-773.
- Burt, A., Carter, D.A., Koenig, G.L., White, T.J., and Taylor, J.W. 1995. Safe extraction of DNA from the human pathogen Coccidioides immitis (Ascomycota). Fungal Genetics Newsletter 42:23. http://www.fgsc.net/fgn42/burt.html
- Bowman, B.H. and Taylor, J.W. 1993. Molecular phylogeny of pathogenic and non-pathogenic Onygenales. Pp. 169-178. In: The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics. D.R. Reyolds and J.W. Taylor (eds.), C.A.B., International, Surrey.
- Bowman, B.H., Taylor, J.W. and White, T.J. 1992. Molecular evolution of the fungi: human pathogens. Molec. Biol. Evol. 9:893-904. https://doi.org/10.1093/oxfordjournals.molbev.a040766